Autologous Bone Marrow Aspirate (BMA) Harvest, BMAC Processing, and MSC Enumeration by Flow Cytometry
Clinical and Scientific Rationale
Autologous bone marrow–derived orthobiologic therapies rely on careful attention to harvest technique, processing methodology, and objective cellular characterization. Mesenchymal stromal cell (MSC)–containing populations are present at very low frequencies within native bone marrow, typically on the order of 0.001–0.01% of total nucleated cells, and their yield is highly sensitive to aspiration technique, processing forces, and operator-dependent variables (Jones et al., 2002; Kraeutler et al., 2021; Hernigou et al., 2005, 2025).
At Boulder Biologics, our approach integrates optimized bone marrow aspiration, controlled preparation of bone marrow aspirate concentrate (BMAC), and quantitative flow cytometric analysis using the Sysmex XF-1600 platform to characterize total nucleated cells, viability, and MSC-enriched subpopulations in live, freshly processed samples.
This page outlines our end-to-end workflow, from BMA harvest to BMAC concentration and MSC enumeration. It illustrates how these steps are combined to support evidence-informed autologous orthobiologic procedures.
Bone Marrow Aspirate Harvest Technique
Bone marrow aspirate is obtained from the posterior superior iliac spine (PSIS) using a multi-site, low-volume aspiration strategy. Rather than drawing large volumes from a single marrow cavity, the needle is redirected between small-volume pulls (approximately 5 mL per draw) to reduce peripheral blood dilution and enrich mononuclear cell fractions with colony-forming unit–fibroblast (CFU-F) potential.
This technique is supported by foundational work demonstrating significantly higher progenitor cell yields with small-volume, multi-pocket aspiration methods than with large-volume single-site draws (Hernigou et al., JBJS, 2005).
Following aspiration, the bone marrow is passed through a micron-scale filter to remove bone fragments and clots while preserving cellular integrity. A defined portion of the filtered aspirate may be retained unprocessed, while the majority proceeds to controlled concentration.
BMAC Processing and Concentration Principles
Bone marrow aspirate concentrate is generated using centrifugation parameters selected to balance effective mononuclear cell enrichment with cell viability preservation. Excessively high g-forces, while capable of increasing apparent nucleated cell density, have been shown to compromise cell membrane integrity and reduce functional viability.
Our processing protocols emphasize:
Moderate centrifugal forces
Preservation of mononuclear cell populations
Minimization of mechanical stress
Maintenance of sterility throughout processing
The result is a BMAC product enriched for mononuclear cells while maintaining high overall viability, consistent with published observations that processing technique is a significant determinant of final cellular quality (Bansal et al., Scientific Reports, 2021).
A residual (RES) fraction, representing the post-collection material remaining in sterile tubes following buffy coat recovery, is also evaluated to understand distribution efficiency and confirm that clinically meaningful cellular populations are retained in the final BMAC product.
Flow Cytometric Enumeration Using the Sysmex XF-1600
Platform Overview
The Sysmex XF-1600 flow cytometer is employed to perform quantitative analysis of live bone marrow samples before and after concentration. Flow cytometry enables objective assessment of:
Total nucleated cell counts
Cell viability
MSC-enriched subpopulations using validated surface markers
Nucleated Cell Counting and Viability
To quantify nucleated cells, aliquots of BMA, BMAC, and RES samples are diluted in buffered solution and treated with a lytic reagent that removes cytoplasmic membranes while preserving nuclei. Forward scatter–based size gating is used to isolate nuclei within a defined micrometer range, allowing accurate calculation of nucleated cell concentrations.
Cell viability is assessed using DNA-binding viability dyes that selectively enter cells with compromised membranes. Viable and non-viable populations are distinguished based on fluorescence intensity, enabling calculation of percent viability for each sample fraction.
Across representative cases, both BMA and BMAC samples demonstrate high viability, reflecting the use of controlled processing conditions.
Identification of MSC-Enriched Populations in Fresh Samples
Unlike culture-expanded cells, freshly isolated bone marrow contains a heterogeneous mixture of hematopoietic, stromal, and progenitor populations. In this context, MSCs are best identified indirectly using flow cytometric marker combinations that correlate with CFU-F activity rather than relying on in vitro expansion phenotypes.
Our live-sample MSC identification strategy emphasizes:
Lineage exclusion (CD45-negative)
Positive expression of CD271 (LNGFR) and/or tissue non-specific alkaline phosphatase (TNAP / MSCA-1)
These markers have been shown to correlate strongly with clonogenic MSC potential in ex vivo bone marrow samples (Quirici et al., Experimental Hematology, 2002; Sobiesiak et al., Stem Cells and Development, 2010). Importantly, markers such as CD73, CD90, and CD105—commonly associated with culture-expanded MSCs—are often downregulated or absent in freshly isolated marrow populations and are therefore not relied upon for primary enumeration.
Using this approach, the Sysmex XF-1600 enables direct comparison of MSC-enriched fractions from native BMA, processed BMAC, and residual layers, providing an internal quality-control metric for concentration efficiency.
Example Patient: BMA vs. BMAC MSC Enrichment
In representative patient analyses, flow cytometry demonstrates:
A marked increase in total nucleated cell concentration following BMAC processing
A proportional enrichment of CD271-positive and TNAP-positive MSC-associated populations
Preservation of high cell viability across BMA, BMAC, and RES fractions
The residual (RES) fraction typically contains substantially lower MSC frequencies, confirming effective recovery into the final BMAC product. These findings are consistent with published observations that MSC yield and frequency increase following appropriate concentration while remaining a small percentage of total nucleated cells (Kraeutler et al., 2021).
Flow Cytometry Figures and Callouts
The following figures illustrate representative flow cytometric analyses of an example patient sample performed on the Sysmex XF-1600 platform. These figures are provided to demonstrate methodology and comparative enrichment rather than to imply fixed dosing thresholds.
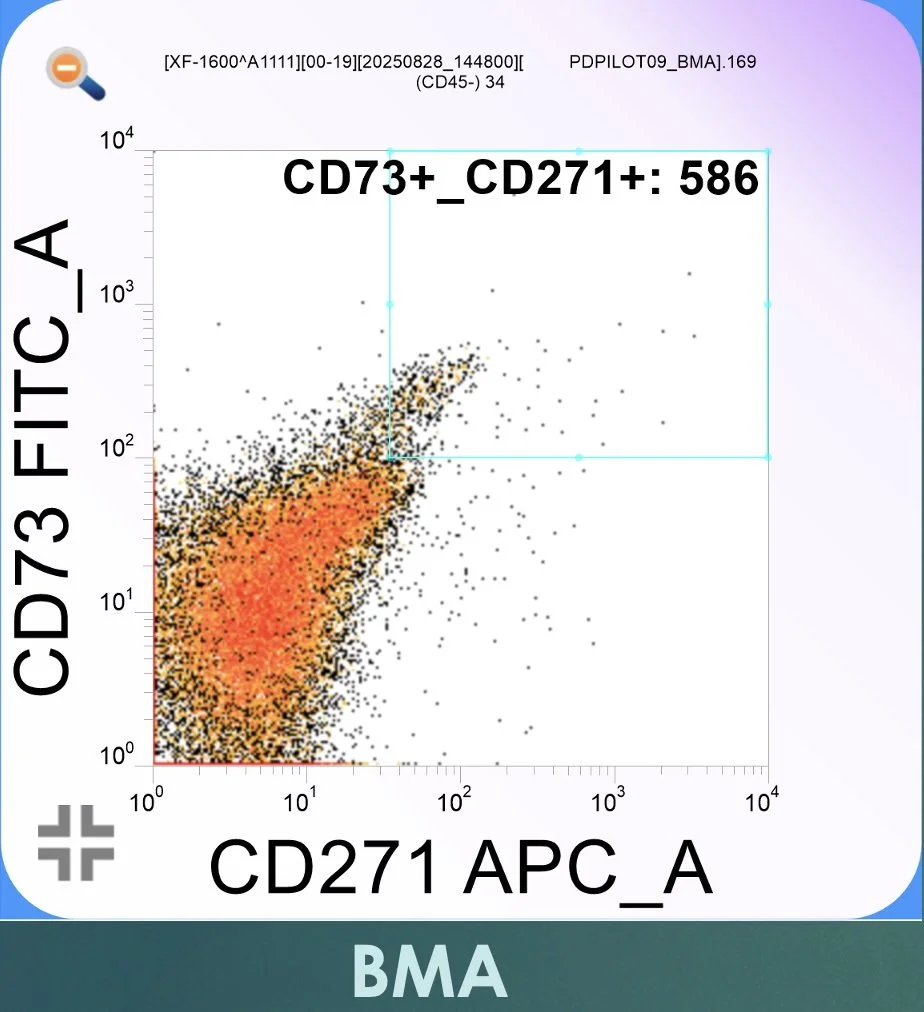
Figure 1. Flow cytometric dot plot from native bone marrow aspirate (BMA) analyzed on the Sysmex XF-1600. CD73-positive events are displayed along the vertical axis (FITC channel), while CD271-positive events are displayed along the horizontal axis (APC channel). Gating is defined by the boxed region in the upper right quadrant, representing lineage-negative, CD73⁺/CD271⁺ events consistent with MSC-enriched populations in freshly isolated marrow.
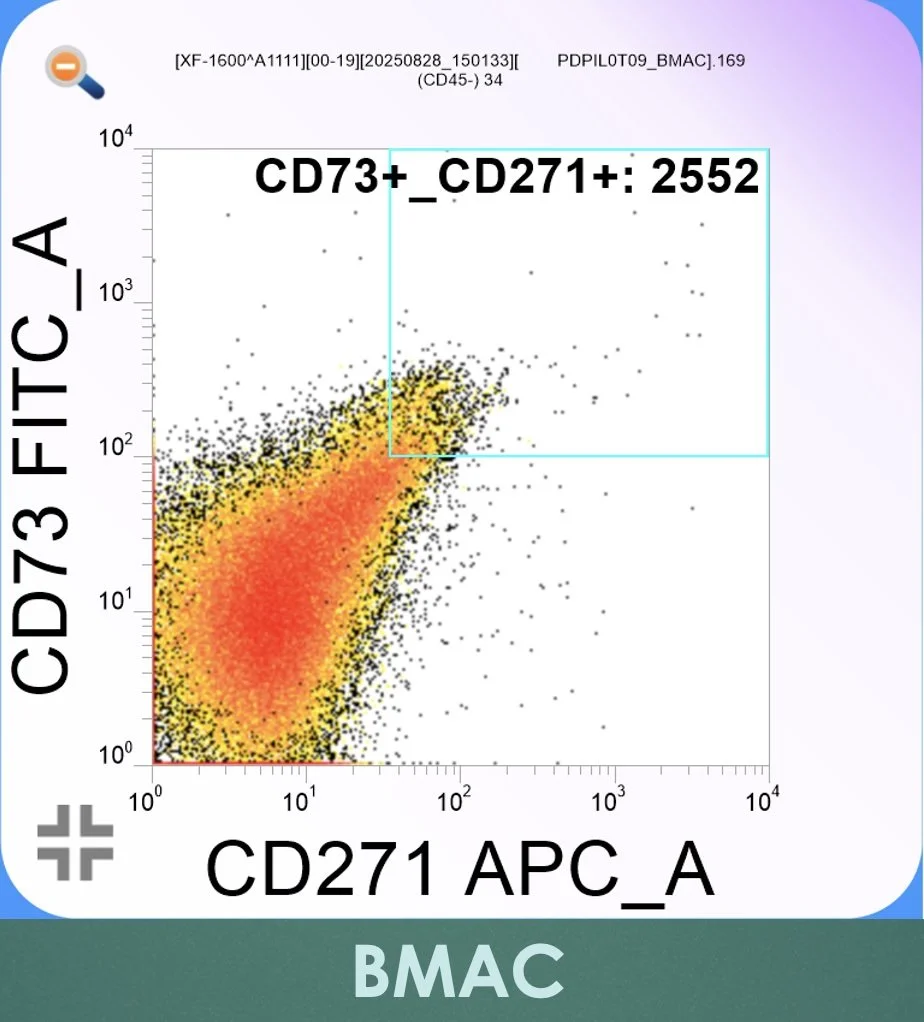
Figure 2. Flow cytometric dot plot from bone marrow aspirate concentrate (BMAC) following controlled centrifugation. CD73-positive events are shown on the vertical (FITC) axis and CD271-positive events on the horizontal (APC) axis. The gated box in the upper right quadrant demonstrates relative enrichment of CD73⁺/CD271⁺ events compared with native BMA, consistent with preferential recovery of MSC-enriched populations while maintaining high cell viability
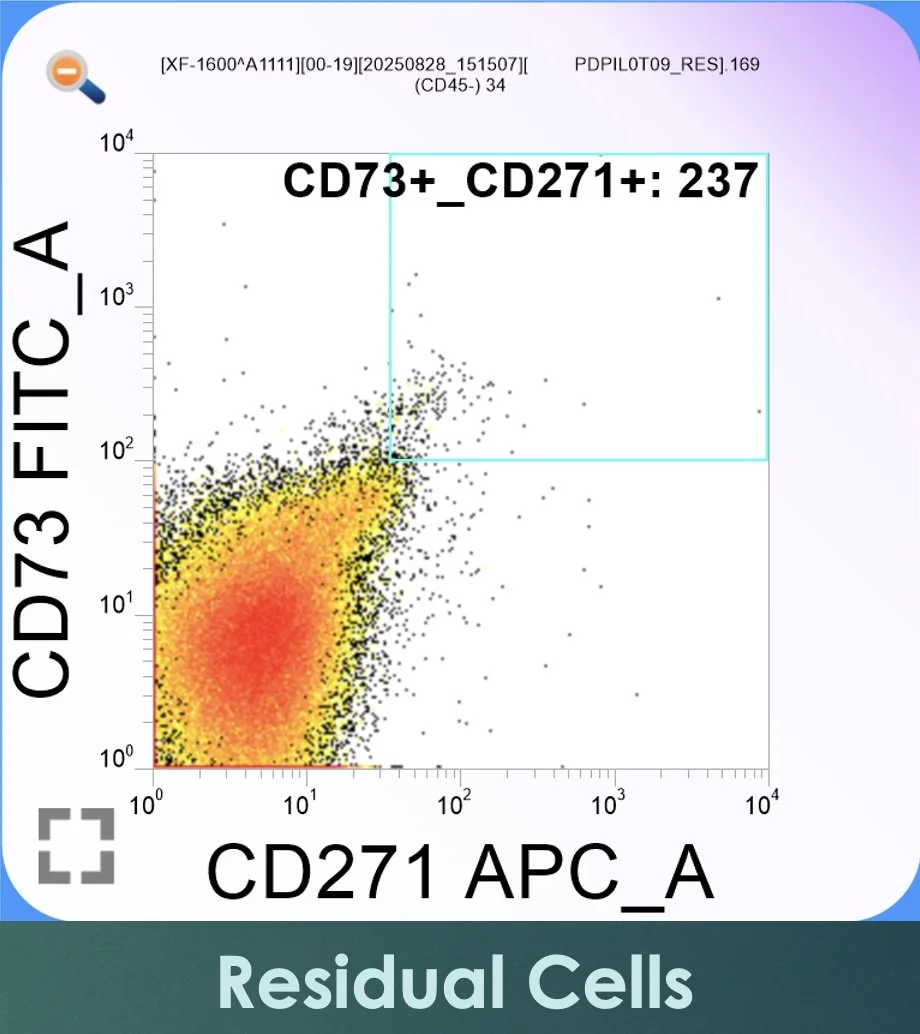
Figure 3. Flow cytometric dot plot from the residual (RES) fraction remaining in sterile tubes following buffy coat collection. CD73-positive events are plotted along the vertical FITC axis and CD271-positive events along the horizontal APC axis. The gated region in the upper right quadrant contains markedly fewer CD73⁺/CD271⁺ events than in BMAC, confirming the effective recovery of MSC-enriched populations into the final concentrate.
Quantitative Comparison of BMA, BMAC, and Residual Fractions
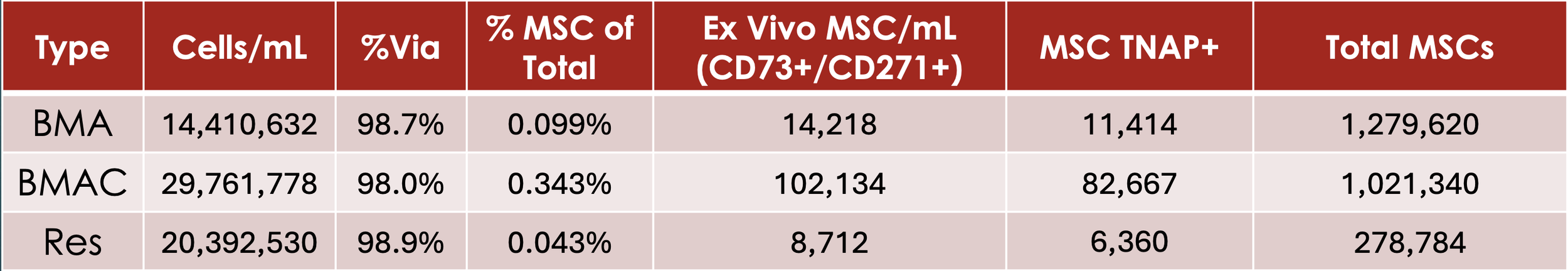
The table below (Figure 4.) illustrates several well-described principles in the orthobiologic literature. First, MSCs represent a very small fraction of total nucleated cells in native marrow, typically well below 1%, consistent with prior CFU-F and flow cytometry studies. Second, controlled BMAC processing increases the relative frequency of MSC-enriched populations despite modest changes in absolute nucleated cell concentration. Third, the residual fraction contains substantially fewer MSC-associated events, confirming effective recovery into the final BMAC product and serving as an internal process-control metric.
Importantly, high viability is preserved across all fractions, underscoring the role of aspiration technique and moderate centrifugation forces in maintaining cellular integrity. These quantitative findings reinforce the notion that meaningful biological enrichment can be achieved without excessive mechanical stress or reliance on unverified assumptions about cellular dose.
Figure 4. Quantitative flow cytometric comparison of native bone marrow aspirate (BMA), bone marrow aspirate concentrate (BMAC), and the residual (RES) fraction following buffy coat recovery. Values were obtained using live-sample analysis on the Sysmex XF-1600 and reflect nucleated cell concentration, viability, and MSC-enriched population metrics.
Clinical Implications
Objective enumeration of nucleated cells and MSC-enriched populations provides transparency and rigor in autologous orthobiologic procedures. By integrating aspiration technique, controlled processing, and validated flow cytometric analysis, Boulder Biologics is able to:
Confirm cell viability and concentration
Quantify enrichment achieved through BMAC processing
Avoid unsupported assumptions about cellular dose
Align clinical practice with contemporary translational research
This approach reflects the growing consensus that orthobiologic therapies should be characterized and reported using measurable cellular parameters rather than relying solely on kit specifications or procedural assumptions.
Regulatory Framework: Same-Day BMAC vs. Culture-Expanded Workflows
Autologous orthobiologic procedures at Boulder Biologics are structured to differentiate same-day bone marrow clearly aspirate concentrate (BMAC) workflows from culture-expanded cellular workflows, consistent with current FDA guidance and established regulatory distinctions.
Same-Day BMA/BMAC Procedures (Clinical Use)
Same-day BMA and BMAC procedures are performed using autologous bone marrow aspirate, which is processed and administered during a single procedural encounter. These workflows are designed to align with the regulatory concepts outlined under Section 361 of the Public Health Service Act and 21 CFR Part 1271, including considerations of:
Autologous use
Minimal manipulation (centrifugation and filtration only)
Homologous use within musculoskeletal tissues
No cell expansion, genetic modification, or prolonged ex vivo culture
In this context, BMAC represents a concentrated, heterogeneous cell-containing biologic product composed of mononuclear cells, hematopoietic progenitors, stromal cells, platelets, and associated signaling molecules. Clinical intent is to support endogenous repair mechanisms through biologically mediated signaling rather than direct tissue replacement or engineered regeneration.
Culture-Expanded Cellular Workflows (Research and Characterization)
Short-term culture and cell expansion workflows are conducted outside same-day clinical care and are used exclusively for research, characterization, and quality evaluation. These workflows involve in vitro culture under controlled laboratory conditions and may result in populations that meet internationally recognized immunophenotypic criteria for mesenchymal stromal cells (e.g., CD73⁺/CD90⁺/CD105⁺).
Culture-expanded cells differ fundamentally from freshly isolated marrow populations in:
Cellular homogeneity
Surface marker expression
Proliferative capacity
Regulatory classification
Accordingly, culture-expanded cells are not administered clinically under same-day protocols and are clearly segregated from patient treatment pathways. This distinction is critical, as surface markers commonly associated with culture-expanded MSCs are often downregulated or absent in native marrow populations and should not be used to infer cell identity or dose in same-day BMAC procedures.
Summary
Autologous BMA harvest, BMAC concentration, and MSC enumeration represent an interconnected workflow. Outcomes depend not on any single step, but on the cumulative effect of aspiration technique, processing forces, and objective cellular validation. Using the Sysmex XF-1600 flow cytometer and evidence-based protocols, Boulder Biologics applies a quantitative, transparent framework for autologous orthobiologic care that reflects current scientific understanding and regulatory requirements