Platelet-rich plasma (PRP)
Platelet-rich plasma (PRP) is an autologous biologic therapy derived from a patient’s own blood and used to support endogenous repair processes in musculoskeletal tissues. PRP consists of plasma with a concentrated platelet fraction, along with associated growth factors, cytokines, and signaling proteins released during platelet activation.
Platelets play a central physiologic role in hemostasis and wound healing. Upon activation, they release bioactive mediators that influence inflammation, angiogenesis, cellular recruitment, and tissue remodeling. When delivered to injured tissues, PRP is intended to modulate the local biologic environment rather than replace damaged tissue, engineer new tissue, or guarantee regeneration.
PRP has been evaluated in a broad range of orthopedic and sports medicine applications, including tendinopathies, ligament injuries, muscle injuries, and degenerative joint disease. Clinical outcomes vary and depend on diagnosis, disease severity, tissue type, PRP formulation, and patient-specific factors.
How PRP Is Prepared and Applied?
Patients should expect a total clinic visit of approximately two hours, with the procedural components typically lasting 10–30 minutes.
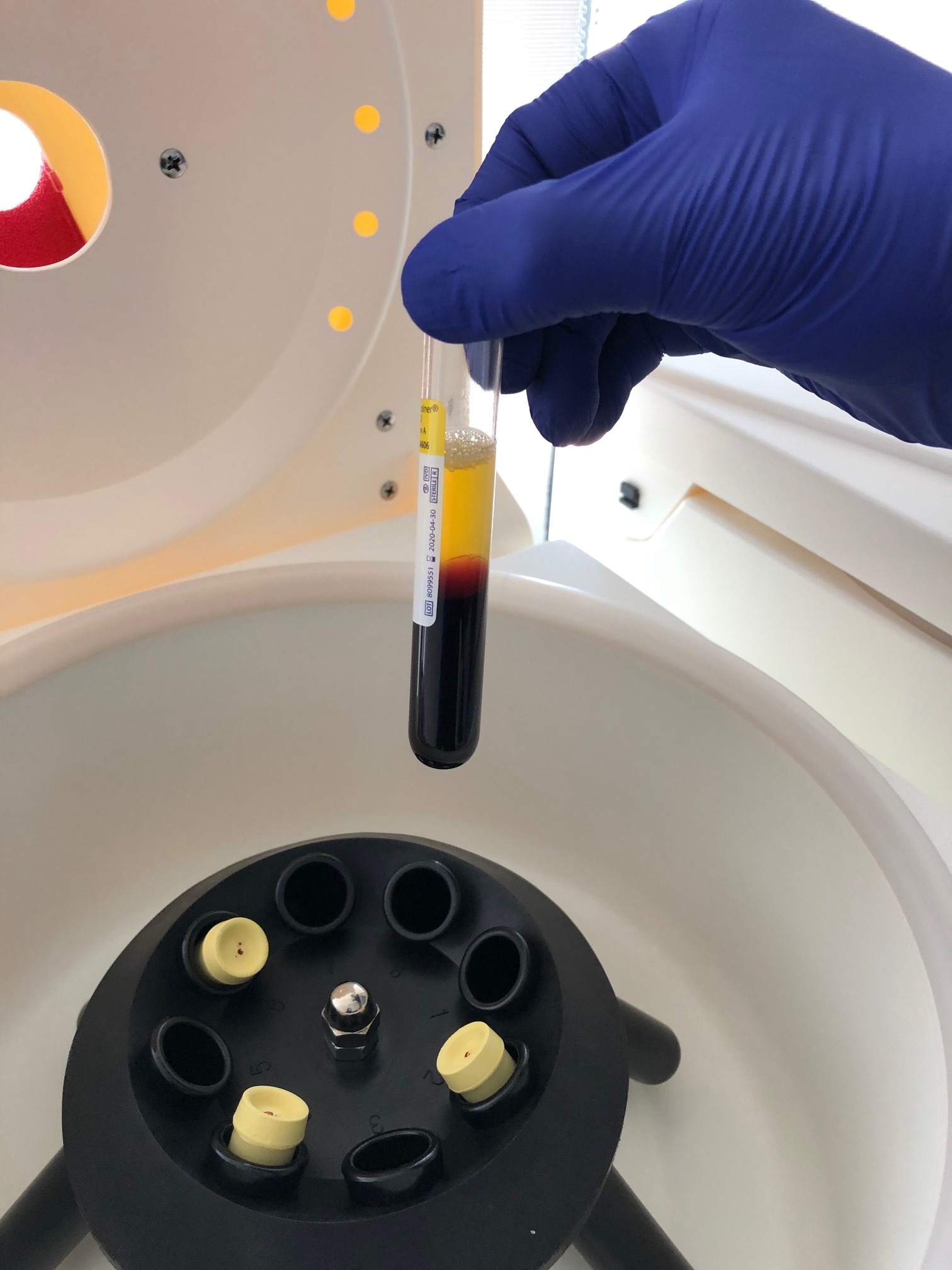
Blood collection: Venous blood is drawn using sterile technique.
Plasma separation: Whole blood is processed in the laboratory to separate plasma from red blood cells using controlled centrifugation.
Platelet concentration: The platelet-rich fraction is refined to achieve a PRP formulation appropriate for the clinical indication. Processing parameters are selected to preserve platelet integrity and biologic activity.
Image-guided administration: PRP is administered by a physician using ultrasound and/or fluoroscopic guidance to ensure accurate anatomic delivery. Non-acidic, non-toxic local anesthetics may be used for patient comfort.
Post-procedure response: PRP is intended to support tissue repair over time through biologically mediated signaling pathways. When benefit occurs, improvement is typically gradual and evolves over weeks to months.
Principles of High-Quality PRP Preparation
Customization of Platelet Concentration
Optimal platelet concentration varies by tissue type and clinical indication. Baseline platelet counts differ substantially between patients due to age, sex, genetics, and health status. In addition, different tissues appear to respond to different biologic signaling profiles.
For example:
Certain peri-neural and soft-tissue applications may benefit from lower platelet concentrations
Tendons and ligaments may tolerate or benefit from higher platelet concentrations
Rather than relying on fixed, pre-packaged kits, Boulder Biologics uses protocol-driven PRP preparation, allowing platelet concentration to be adjusted based on patient- and indication-specific considerations. Baseline platelet counts are assessed using a hematologic analyzer prior to PRP preparation.
Platelet Isolation and Viability
Centrifugation parameters (speed, duration, acceleration) directly affect platelet integrity. Excessive centrifugal forces may damage platelets and reduce their functional capacity. Our protocols emphasize controlled centrifugation forces designed to preserve platelet viability and biologic activity.
Red Blood Cells and Leukocytes
For many orthopedic applications, PRP formulations with low red blood cell and low leukocyte content are preferred. Red blood cells and specific leukocyte populations may contribute to post-injection pain and inflammation. Our processing methods allow selective reduction of red blood cells and leukocytes when clinically appropriate, while maintaining platelet enrichment.
Evidence Base and Clinical Context
The clinical literature consistently emphasizes that PRP outcomes depend on formulation, indication, and patient selection. Multiple consensus statements and classification systems have been developed specifically to address PRP heterogeneity and improve scientific reporting, including the PAW and DEPA classification frameworks.
Systematic reviews and meta-analyses suggest that PRP may improve pain and function in selected musculoskeletal conditions, particularly knee osteoarthritis and certain tendinopathies, but demonstrate significant variability across studies. These findings underscore the importance of formulation transparency, appropriate indication selection, and patient counseling.
PRP should be viewed as a biologically supportive intervention that may complement rehabilitation, activity modification, and comprehensive musculoskeletal care, rather than as a standalone or curative treatment.
Figure A. Baseline complete blood count (CBC) prior to PRP preparation, demonstrating the patient’s starting platelet count and leukocyte/red blood cell indices. Baseline hematologic values are used to inform PRP preparation targets and document patient-specific starting conditions.
Figure B. Post-processing cellular analysis of the prepared PRP product, demonstrating platelet enrichment relative to baseline and documenting red blood cell and leukocyte content. PRP composition varies by formulation and indication and is clinically relevant, as PRP is not a single uniform product.
Figure C. First centrifugation step (“soft spin”) during platelet-rich plasma preparation. Whole blood is centrifuged using controlled, low-force parameters to separate platelet-rich plasma from red blood cells and the majority of leukocytes. This step allows plasma containing suspended platelets to be isolated while minimizing platelet activation and preserving cellular integrity.
Figure D. Second centrifugation step (“hard spin”) during platelet-rich plasma preparation. The platelet-rich plasma fraction undergoes higher-force centrifugation, resulting in the concentration of platelets as a visible pellet at the bottom of the conical tubes. Following this step, excess platelet-poor plasma is removed, and the platelet pellet is resuspended to achieve a PRP formulation appropriate for the clinical indication.
Regulatory Disclaimer
Platelet-rich plasma is an autologous biologic product prepared from a patient’s own blood. PRP has not been approved by the U.S. Food and Drug Administration (FDA) for the treatment of specific diseases or musculoskeletal conditions. Clinical use is based on physician judgment, current scientific evidence, and individualized patient evaluation. No claims are made regarding guaranteed outcomes, tissue regeneration, or disease modification.
Selected References
DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Am J Sports Med. 2012;40(4):106–114.
Magalon J, Chateau AL, Bertrand B, et al. DEPA classification: a proposal for standardising PRP use. BMJ Open Sport Exerc Med. 2016;2:e000060.
Dohan Ehrenfest DM, et al. Classification of platelet concentrates. Trends Biotechnol. 2014;32(1):21–32.
Hurley ET, et al. Expert consensus on PRP treatments for musculoskeletal pathologies. Arthroscopy. 2024.
Costa LAV, et al. PRP versus other therapies for knee osteoarthritis: a meta-analysis. Am J Sports Med. 2023.
Oeding JF, et al. PRP versus hyaluronic acid for knee osteoarthritis: systematic review and meta-analysis. Am J Sports Med. 2024.
Hamid MSA, et al. PRP for rotator cuff tendinopathy: systematic review and meta-analysis. PLoS One. 2021.