Autologous Orthobiologic Therapy for Orthopedic Applications
Boulder Biologics provides autologous, patient-derived orthobiologic procedures intended to support the body’s endogenous repair processes in acute and chronic musculoskeletal injuries. These interventions utilize minimally manipulated biologic products derived from a patient’s own bone marrow and/or adipose tissue and are delivered using image-guided techniques for precision and safety.
Rather than relying on unsupported claims of cell replacement or differentiation, our clinical approach is grounded in the current scientific understanding that mesenchymal stromal cell–containing biologic products exert their effects primarily through paracrine signaling, immunomodulation, and support of local tissue repair environments.
Mesenchymal stromal cell–enriched populations derived from bone marrow or adipose tissue are known to secrete cytokines, chemokines, extracellular vesicles, and growth factors that may influence inflammation, angiogenesis, and tissue remodeling. These biologic signals can be particularly relevant in musculoskeletal tissues such as cartilage, tendon, ligament, bone, and muscle, which often demonstrate limited intrinsic healing capacity following injury.
Autologous biologic therapies deliver a concentrated biologic milieu to injured tissues, aiming to augment the local repair response rather than directly replace damaged cells. Bone marrow–derived and adipose-derived biologic products differ in cellular composition and signaling profiles, and clinical selection is based on injury type, location, and patient-specific factors.
Conditions commonly evaluated for autologous orthobiologic treatment include:
Osteoarthritis
Bone marrow edema
Avascular necrosis of bone
Fracture nonunion or delayed union
Tendon injuries
Ligament injuries
Articular cartilage injuries
All treatments are individualized following clinical evaluation and imaging review.
Overview of the Autologous Orthobiologic Procedure
Check-in and blood collection
Upon arrival, patients undergo intake and venous blood collection for preparation of autologous platelet-rich plasma (PRP). PRP preparation is described in detail in the PRP section of this website.
PRP preparation
In our laboratory, whole blood is processed to separate plasma components and concentrate platelets, growth factors, and signaling proteins. The resulting PRP is prepared for image-guided administration.
Autologous tissue harvest
A small volume of bone marrow aspirate is obtained from the posterior superior iliac spine (PSIS). When clinically appropriate, a limited adipose tissue harvest is also performed from the flank region. Both harvests are conducted using sterile technique and local anesthesia.
Biologic processing
Collected autologous tissues are processed to concentrate cell-containing and acellular biologic fractions appropriate for clinical use. Processing is performed using standardized protocols designed to preserve viability and sterility while remaining consistent with applicable regulatory guidance.
Image-guided administration
Using ultrasound and/or fluoroscopic guidance, autologous PRP and cell-containing biologic products are delivered to the targeted tissue or anatomic compartment. Image guidance is used to optimize accuracy, minimize risk, and ensure appropriate localization.
The injected autologous biologic material is intended to support tissue repair over time through biologically mediated mechanisms.
Patients remain awake during the procedure. We may supply them with a prescription sedative like Valium if they elect to take it. Many patients manage the procedure without a sedative.
Though patients remain in the clinic for three to four hours, much of that time is spent preparing their PRP and stem cells in our lab. The harvest takes 20-30 minutes and the delivery takes 15-45 minutes.
FAQs and more information about the procedures (both the harvest and injections) are included at the bottom of this website page.
Procedural Experience and Timing
Patients remain awake during all procedures. Oral anxiolytic medication may be prescribed at the patient’s discretion, though many patients tolerate the procedure without sedation.
Total clinic time is typically three to four hours, primarily due to biologic preparation and processing.
Tissue harvest: approximately 20–30 minutes
Image-guided delivery: approximately 15–45 minutes, depending on injury location and imaging modality
Post-procedure care instructions are reviewed in detail following completion.
Boulder Biologics’ Clinical Approach
Our approach to autologous orthobiologic therapy emphasizes:
Individualized selection of biologic products based on injury type and severity
Quantitative assessment of cell counts and viability when appropriate
Use of advanced imaging (ultrasound and fluoroscopy) for procedural precision
Controlled laboratory environments and biosafety cabinets to maintain sterility
Strict adherence to evidence-based practice and regulatory-aware terminology
Dr. Glowney has extensive experience performing image-guided orthobiologic procedures across a broad range of musculoskeletal conditions.
Bone Marrow and Adipose Tissue Harvest
After skin antisepsis, local anesthetic is administered over the posterior iliac region. A specialized needle is used to obtain bone marrow aspirate from the PSIS, with ultrasound guidance employed when clinically indicated.
If adipose tissue harvest is appropriate, a small incision is made in the flank region, and a limited volume of adipose tissue is obtained. The combined harvest process typically requires 20–30 minutes.
Image-Guided Biologic Injections
Injection sites are disinfected and locally anesthetized using non-toxic anesthetic agents. Ultrasound or fluoroscopic guidance is used to deliver autologous PRP and cell-containing biologic material to the targeted tissue.
Injection time varies depending on anatomy and imaging modality, but generally ranges from 15 to 45 minutes. Aftercare instructions and activity guidance are reviewed with the patient upon completion.
Frequently asked questions and additional procedural details are available at the bottom of this page. For consultation inquiries, we encourage patients to contact our office directly.
Orthobiologic Therapy FAQ
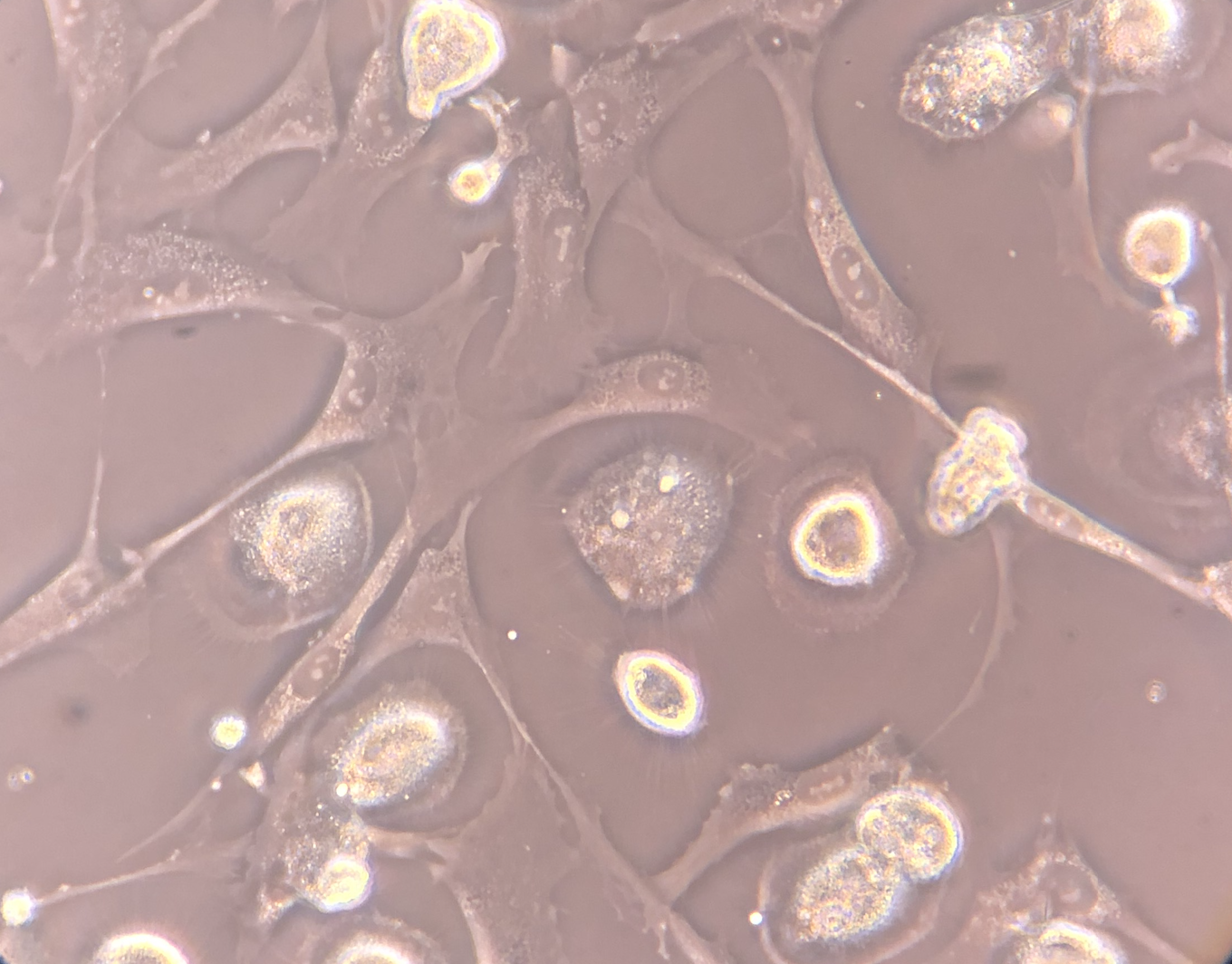
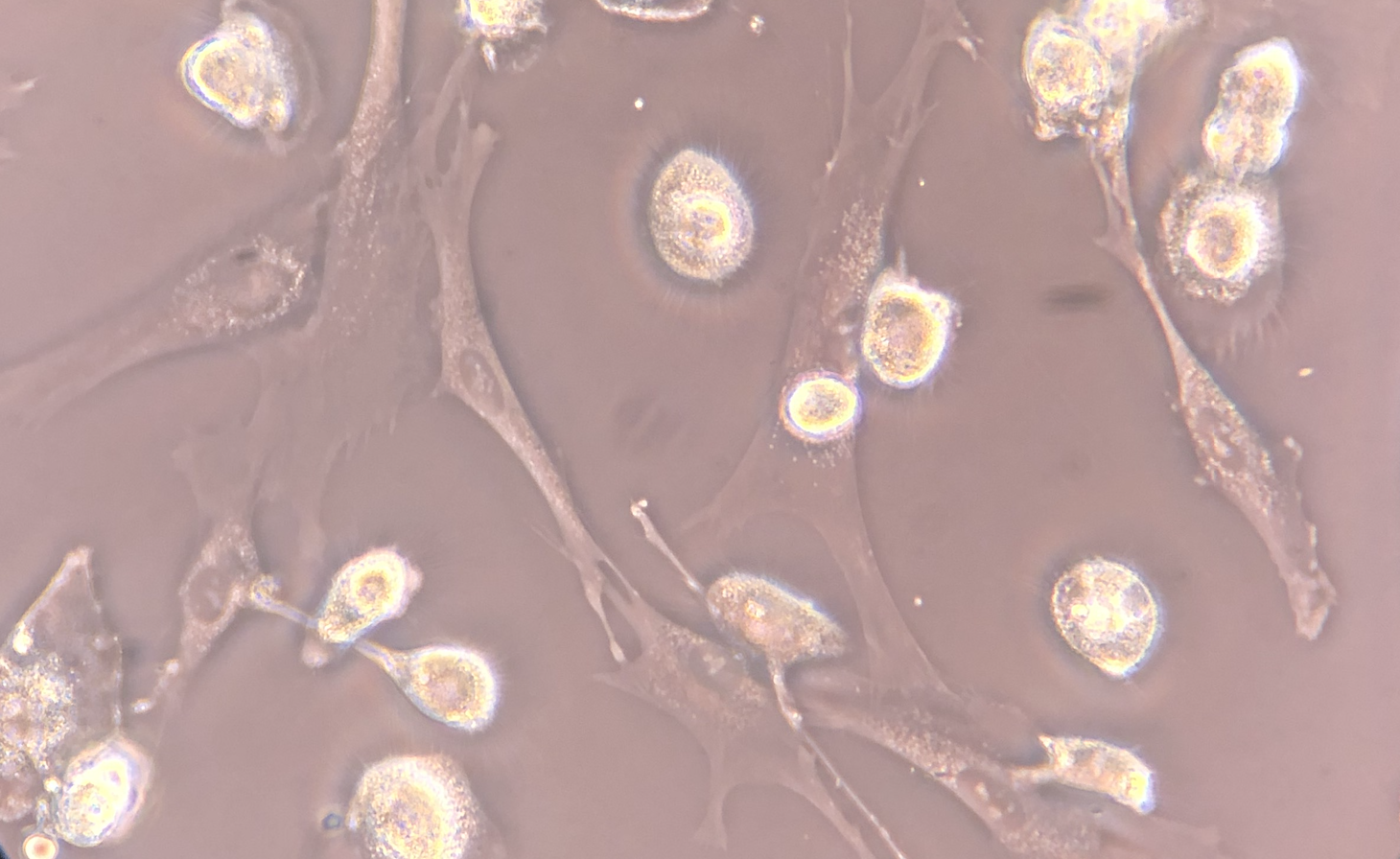
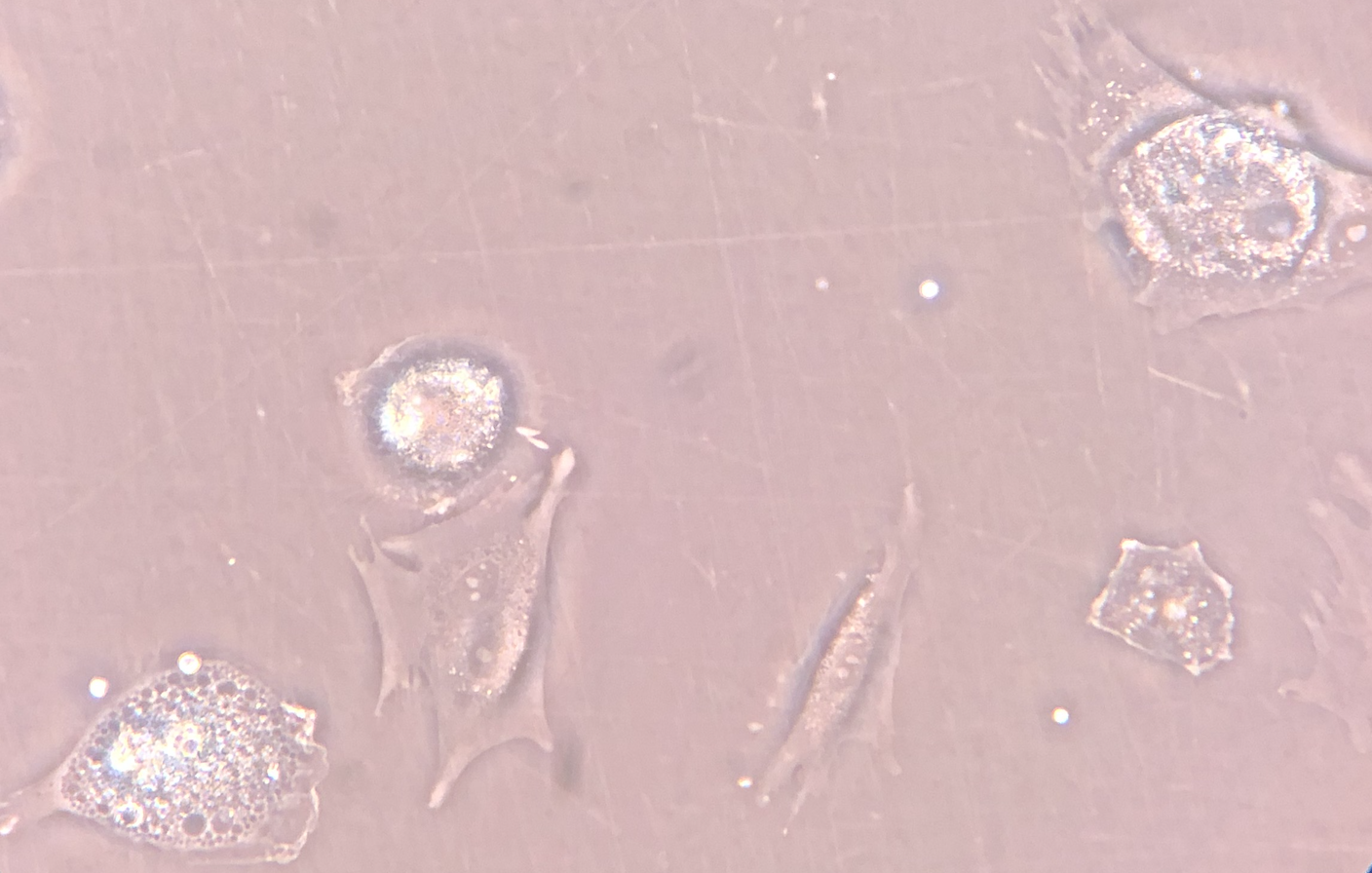
Mesenchymal Stromal Cell (MSC) Culture – Phase-Contrast Microscopy - Phase-contrast microscopy images demonstrating adherent, fibroblast-like stromal cells derived from human bone marrow under controlled in vitro culture conditions. Images 1 and 2 show proliferating adherent stromal cells with characteristic spindle-shaped morphology and visible daughter cells during short-term expansion. Image 3 demonstrates early plastic adherence approximately two hours following passaging, a defining phenotypic property of mesenchymal stromal cells in culture.
These images represent laboratory-based cell characterization and illustrate established MSC culture behavior, including adherence to tissue culture plastic and proliferative capacity under appropriate conditions. The culture-expanded cells shown are for research, quality assessment, and phenotypic documentation purposes only and are not representative of the same-day, ex vivo autologous biologic products used clinically.

